
Lithium isooctanoate has its roots in the broader exploration of metal carboxylates, where scientists in the mid-twentieth century began focusing on lithium salts to harness their beneficial effects in various industries. Chemical engineers, working on improving lubricants and specialty chemicals, noticed the performance differences lithium-based compounds brought about when compared to calcium or sodium salts. Isooctanoic acid itself saw significant industrial activity during the push for synthetic lubricants in the 1960s and 70s. Lithium was already a staple in pharmaceutical and battery research, and combining it with medium-branched carboxylic acids opened new doors, especially in applications demanding stability under tough operating conditions. By the 1980s, research into lithium carboxylates, including isooctanoate, shifted from bulk lubricant use into specialty and fine chemistry areas, surfacing in polymer manufacturing, electronics, and antimicrobial fields. Manufacturers shifted from batch-style, small-scale synthesis to more refined production, driven by environmental and efficiency policies in Europe, North America, and Asia.
Lithium isooctanoate stands out as a specialty salt, produced by neutralizing isooctanoic acid with lithium hydroxide or lithium carbonate. It typically appears as a colorless, sometimes faintly yellow solution or low-melting solid, depending on purity and processing. This compound supports diverse sectors. Paint and coatings technologists find it valuable because it speeds up drying reactions in alkyd resins, while researchers in electronics rely on lithium isooctanoate for its role in conductive polymers and dopant blends. Its chemical structure builds on a branched C8 backbone, which brings both hydrophobicity and thermal resistance — features that end up quite useful in automotive additives, cable manufacturing, and certain biomedical technologies.
This compound settles somewhere between a solid and a viscous liquid at room temperature, depending on its specific hydration or solvent load. Most forms supply a moderate-to-low melting point, often in the range of 35°C to 45°C, which eases blending with oils or polymeric batches. Lithium isooctanoate dissolves readily in alcohols and certain glycols, but resists dispersing in water. The branched isooctanoate group blocks effective crystal stacking, so chemical engineers value it for making waxy, easily worked mixtures. Lithium, sitting as the active cation, gives the salt unusual thermal and chemical resistance. Subtle but useful alkaline reactivity makes it helpful for modifying curing rates and balancing acid numbers in organic formulations.
Specification sheets often demand a lithium content somewhere around 2.5–3.5% by weight, with the vast majority of the compound as the carboxylate salt and minimal leftover acid or base. Purity grades register above 98%, with limits on heavy metals and halides set by both environmental and industrial standards. Suppliers label containers with UN codes, hazard data, and expiration dates, due to light sensitivity and mild corrosivity. Details such as particle size (for solid forms), water content, and solvent compatibility guide formulators, especially for paint, adhesive, or electronics uses, since small changes in water or hydrocarbon content shift downstream performance. Packaging typically uses lined drums or high-density polyethylene containers to block moisture and chemical breakdown.
Most industrial synthesis routes start by blending purified isooctanoic acid with either lithium hydroxide or lithium carbonate. Operators work in enclosed reaction vessels (usually under nitrogen) and mix at moderate temperatures, often below 60°C to avoid acid volatilization or salt decomposition. Process control focuses on slow addition, maintaining a slight excess of lithium source to scavenge residual acid and drive the reaction to full neutralization. Once the salt forms, workers purge water either by vacuum or gentle heating, concentrating to the desired solid content or fluidity. Final preps may pass through filtration to snag traces of insoluble grit, followed by controlled cooling to collect dry flakes or a viscous bulk liquid for shipping and downstream use.
Once manufactured, lithium isooctanoate participates in a range of chemical processes, supporting both modification and integration into other materials. In coatings and inks, this salt reacts with polyol esters and alkyds to catalyze curing, helping to set films quickly without forming brittle layers. Material scientists retrofit lithium isooctanoate by exchanging the lithium cation with other metals or doping the molecule into larger constructs, tuning properties such as solubility, melting point, or reactivity. Some polymers, notably those based on ethylene-vinyl acetate or certain acrylates, anchor the isooctanoate units directly, improving resistance to hydrolysis and sunlight-driven aging. Reactive extrusion and solution blending allow manufacturers to incorporate this salt in both solid and liquid products, providing consistent distribution without unwanted clumping or oversaturation.
Across the chemical supply chain, this salt goes by several names. Lithium 2-ethylhexanoate ranks as the most recognized technical synonym, echoing the alternative name for the acid precursor. Some catalogs list it simply as lithium octanoate or lithium C8 carboxylate. In German and Japanese literature, translations of lithium isononanoate sometimes appear, but these can be misleading—actual isononanoic acid is a structural cousin rather than a true match. Trade products sometimes brand the salt with nickname abbreviations, such as "LIOCTA" or "L-2EH," and plenty of regional suppliers market a proprietary spin, which can cause some confusion among buyers and formulators navigating regulatory reviews or cross-border procurement.
Even at moderate concentrations, the compound deserves careful handling. The lithium ion has recognized biological activity and requires restrictions in both worker exposure and waste management, set out by REACH, OSHA, and analogous Chinese or Japanese authorities. Material safety data sheets call for gloves, goggles, and splash-resistant lab coats, particularly where hot melts or concentrated solutions hit routine use. Spill protocols specify inert absorbents, ventilation, and disposal under hazardous waste codes if bulk quantities break containment. In manufacturing plants, suppliers maintain emergency eye wash and surplus containment drums. Fire risk stays low due to the high flash point, but incomplete combustion can produce toxic organic vapors. Transport relies on strict labeling, avoiding contact with strong oxidizers or acids that might trigger violent release of heat or irritating gases.
Paints and alkyd-based finishes count on lithium isooctanoate for faster cure times and enhanced film durability, competing with calcium or cobalt salts without introducing heavy metals. Electronics manufacturers blend it into specialty polymers for improved dielectric properties, tuning electrical response in components that power everything from automotive sensors to solar modules. Antimicrobial research teams employ it for surface treatments that inhibit bacterial adhesion, leveraging both the lithium cation and fatty acid structure to disrupt microbe membranes. In rubber compounding and lubrication, the salt builds stable, non-toxic thickeners that hold up under thermal or shear stress—critical for EV battery seals, wind turbine gearboxes, and aerospace bearings. Some advanced ceramics and glasses integrate this compound to tweak optical responses or density, supporting the drive for lighter, more effective components in communications and imaging tech.
R&D groups press ahead with new ways to use and modify lithium isooctanoate, with considerable activity tied to green chemistry. Teams at universities and private firms alike look for new catalyst systems that cut out toxic metals in coatings, aiming for sustainable, high-performing films and varnishes. Polymer scientists experiment with grafting the isooctanoate structure onto block copolymers or as ionic side chains, pushing flexibility, chemical resistance, or self-healing properties. In electronics, electrospinning and wet chemistry routes incorporate the salt into fibers and films for lightweight, flexible batteries and sensors. Patent trackers notice a growing list of filings on hybrid coatings with embedded lithium salts, targeting water resistance, color stability, and anti-microbial effects. Collaborative projects often bring together paint makers, rubber companies, and electronics firms, driving custom blends for each sector’s rigorous demands.
Toxicological reviews underline differences between lithium isooctanoate and simple lithium salts such as carbonate or chloride. The ester-derived acid component, widely used in food contact and cosmetic ingredients, carries a low acute toxicity, supported by rodent and in vitro studies showing limited absorption or biological disruption. Researchers studying chronic lithium exposure flag a need for caution at high doses, referencing biomonitoring data for workers in manufacturing. No evidence yet ties regular, controlled use of lithium isooctanoate to significant carcinogenic or mutagenic effects, and environmental impact studies suggest the branched acid breakdown proceeds at a rate and pathway comparable to other short-chain fatty acids. Some scrutiny remains on cumulative lithium load in aquatic settings, given the metal’s bioavailability and persistence, so new research frequently tracks leaching, breakdown, and potential bioaccumulation across product life cycles.
Looking ahead, the outlook for lithium isooctanoate cleaves closely to growth in specialty chemicals and the steady evolution of greener, high-performance industrial formulas. Demand for non-cobalt driers in paints continues to expand as regulations grow tighter on heavy metals and volatile organics. Electric mobility and electronics packaging motivate polymer blends that demand just the sort of stability and conductivity lithium carboxylates offer, and the shift to renewable energy keeps boosting demand for safer, longer-life lubricants and sealants. Researchers identify plenty of opportunity in surface science, especially antimicrobial coatings and sustainable, low-VOC adhesion systems. Chemical manufacturers invest time and resources into scaling efficient, waste-minimized production while synthetic biologists survey whether alternative, bio-derived routes to isooctanoic acid can cut the carbon footprint. Academic labs keep pressing into variance in cation choice, chain branching, and hybridization, capitalizing on lithium isooctanoate’s flexibility and the market’s appetite for next-generation solutions that balance safety, sustainability, and technical muscle.
Walk into any industrial plant with heavy gears spinning, and you’ll spot maintenance engineers eyeing the lubrication systems like hawks. If you dig a little deeper, you’ll find that lithium-based greases keep those machines running, and lithium isooctanoate plays a supporting role. It helps create greases that don’t lose their texture or break down even when temperatures soar. Mechanics depend on these products because equipment downtime drives up costs and disrupts production. Without additives that lift grease performance, you’re left scraping failed residue off axle housings and trying to figure out why parts keep grinding.
Chemists count on lithium isooctanoate to boost certain properties in lubricants. It helps soaps blend with oils, feeding the texture and consistency users expect from a dependable grease. There are plenty of choices for thickening greases, but lithium-based ingredients still carry the crown for service in trucks, tractors, and buses. Lithium isooctanoate forms stable soap structures that hold up during rain, heat, or pressure. That means semi-truck fleets and farm combines can run long days without mechanics scrambling to reload bearings or scrape out sludge.
Rust chews through metal in humid or salty conditions, but lithium isooctanoate delivers another helpful trick — corrosion resistance. Slather gear teeth or rolling joints with a grease packed with lithium compounds, and you buy yourself days or weeks before rust gets a foothold. There’s a reason that factories in port cities or paper mills lean on these formulations. Salt and moisture soak the air, putting machinery at risk. A well-formulated lithium grease cuts down both breakdowns and routine maintenance for many operators.
Lithium isooctanoate also graces the green movement, at least in part. Specialty lubricants today compete on both performance and environmental safety. Chemists are looking at alternatives that break down more easily or rely on less energy to produce. The lithium content comes from mining, and that has its own impact, but the continued push for cleaner, safer grease technology delivers progress. Factory managers keep one eye on effluent standards and regulatory shifts, so a lubricant that lasts longer between changes sends less waste toward treatment plants. Reducing oil changes means fewer spills, less landfill, and lower hazardous waste.
Suppliers juggle price swings for lithium, which links the additive to world market trends. Bumps in raw material supply can make prices jump, which hits budgets across manufacturers and service firms. End users sometimes feel it when product prices leap, so balancing cost with reliable performance never gets simple. Some companies try blends with less lithium content, but few surpass the track record of classic lithium soaps boosted with additives like isooctanoate.
New technology keeps rolling in, with plant-based thickeners and advanced additives offering alternatives. Still, in heavy-duty shops across the country, lithium isooctanoate helps prevent breakdowns and extends life in a way mechanics and engineers can count on. Testing new blends remains slow, given the trust built around proven solutions.
Lithium isooctanoate isn’t a household name, yet it plays a behind-the-scenes part in keeping essential machines and vehicles in shape. Reliable equipment means fewer delays and better safety. Looking ahead, the search for less harmful, longer-lasting additives rolls on, and field experience is what pushes real progress.
Lithium isooctanoate pops up in a surprising number of places, from specialty chemicals and lubricants to batteries. Once this compound enters the shop or lab, safety quickly turns into a team effort. Breathing in fumes or getting skin exposed adds up, not just in short-term discomfort but also long-term health concerns. Common mistakes catch up with people, like not using gloves or skipping a face shield just for a “quick job.”
Many industrial chemicals have quirks, but lithium compounds often cause direct irritation. Even without a spill, fumes can sneak up on folks. A few minutes of exposure may not feel like much at first, yet repeated low-level contact tends to dry out skin, spark rashes, or aggravate lungs. Once, a former colleague dismissed mild redness after a splash, brushing it off until his hands started peeling days later. The lesson hit home—safety gear isn’t optional, even if the job looks routine.
Ventilation forms your first line of defense. Keeping fans humming and hoods running goes beyond just moving air. A good ventilation setup means fumes have less chance to build up, and the workspace stays clear. Relying on a gut feeling can backfire, so periodic checks on air quality and airflow serve as a smart backup. Forgetting to swap a clogged filter may mean trouble later, so a maintenance schedule makes practical sense.
Personal protective equipment—PPE—matters as much as the tools collected for a task. Nitrile gloves, safety goggles, and lab coats create a barrier. Eye protection stands out, since one splash to the eye can set recovery back for weeks. Respirators sometimes get ignored for “quick” chores, but chemical safety doesn’t play by those rules. Changing gloves and masks often, before they wear thin, saves headaches.
Storing lithium isooctanoate in tightly sealed containers keeps moisture and air out. Exposure to water or acids sometimes triggers chemical changes or releases harmful fumes. Keeping this chemical in clearly labeled containers, tucked away from high-traffic spots, prevents confusion or cross-contamination. Regular inventory checks help spot leaks or damaged seals before they grow into a bigger problem. At my last shop, a corroded lid nearly led to a spill—catching it during a weekly review stopped trouble in its tracks.
Spills occasionally happen, no matter how careful the team is. Having a spill kit nearby, stocked with absorbent materials, offers peace of mind. Teams working with hazardous materials run regular drills so that everyone knows their role. Relying on memory or skipping walkthroughs can turn a small leak into a serious mess. A culture of speaking up about safety—no matter how small the mistake—encourages everyone to stay alert.
Proper disposal rounds out the process. Dumping leftover lithium isooctanoate down the drain isn’t just bad for the environment; it breaks local laws and could invite big fines. Designated hazardous waste containers guarantee chemicals don’t mix where they shouldn’t. Certified waste haulers handle pick-up and disposal—paying a little more means fewer headaches down the line.
Trust builds inside workplaces that stick with good safety habits. Training shouldn’t stop after orientation; regular refreshers keep knowledge sharp. Publishing near-miss stories—instead of hiding them—lets everyone learn without judgment. Safety wins show up not in the number of accidents, but in the confidence teams bring to every shift.
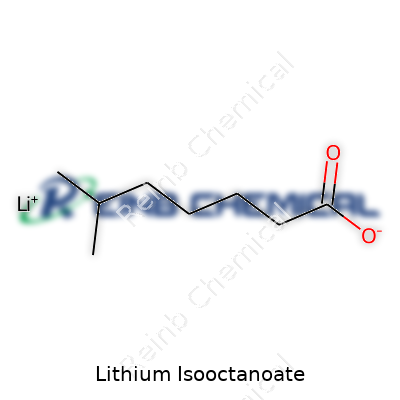
Lithium isooctanoate sounds like something pulled straight from a shelf in a lab, but it’s just the lithium salt derived from isooctanoic acid. Chemists know this acid by another name: 2-ethylhexanoic acid. It has a backbone of eight carbons twisted in a way that earned it the “iso” descriptor.
Write the formula for isooctanoic acid and you get C8H16O2. Replace the hydrogen on the carboxyl group with lithium, and lithium isooctanoate becomes C8H15LiO2. That single change opens the door for applications beyond the chemistry textbook.
Anyone who has worked with specialty lubricants or additives knows the importance of lithium compounds. Lithium isooctanoate finds a home in these markets. Companies working on metal soaps like lithium stearate or lithium 12-hydroxystearate often branch out to lithium isooctanoate when a particular performance tweak is needed. Some anti-corrosive lubricants benefit from C8H15LiO2, especially where standard lithium greases fall short with high temperatures or water resistance.
Research from the Society of Tribologists and Lubrication Engineers shows demand for specialty lithium salts has climbed with the spread of advanced machinery. Publicly available safety sheets detail how lithium isooctanoate supports stability in greases, harsh-temperature lubricating oils, and certain plasticizers, all thanks to its reliable chemistry.
No one wants to guess their way around an MSDS, especially with workplace exposure on the line. Knowing the exact formula—C8H15LiO2—means teams handle and store compounds with proper respect for fire risk, toxicity, and reactivity. One wrong item in the storage area puts both product quality and worker safety at risk. Getting the chemical formula right isn’t about showing off lab skills—it’s about clear communication in production environments, safe protocols, and trust downstream.
Lithium isooctanoate also paves the way for more sustainable solutions. Its solubility profile and low volatility allow chemists to formulate lubricants that are less likely to vaporize or contaminate the workplace. Many processes shift to specialty salts to lower emissions and to meet ever-stricter environmental codes. Watching companies invest in plant upgrades to accommodate less hazardous lithium salts highlights the impact a simple chemical formula can have far beyond paperwork.
Supply chain hiccups snap into focus with demand for lithium salts rising. The lithium industry faces pressure from both battery manufacturers and specialty chemical producers, so the purity and consistency of materials like lithium isooctanoate hold new importance. Contamination or batch errors hurt not just one line of products but an entire manufacturing chain. Certifications from organizations such as ISO or REACH become key, offering peace of mind for buyers and accountability for suppliers.
As clean technology carves a larger slice out of lithium supply, alternatives and improved recycling methods will become central. For now, lithium isooctanoate remains a go-to for engineers and formulators who value its unique blend of chemical stability and performance.
Lithium isooctanoate pops up in the world as a specialty salt, often used as a lubricant additive or as part of grease formulations. As the demand for new battery technologies and different forms of lithium swells, alternative lithium chemicals like this one ride that wave. I’ve worked with chemical product safety data long enough to know that when something with the word “lithium” shows up, you can’t assume it’s harmless. We’ve all seen the headlines: batteries catch fire, raw material mining scars the landscape, and certain industrial chemicals end up in unexpected places.
You won’t see lithium isooctanoate spilling into the news like oil slicks do, but its risks can’t be ignored. The compound doesn’t explode on contact with water, and it doesn’t come with strong fumes. That sounds reassuring until you realize that subtle, ongoing leaks are just as insidious as the headline-grabbing disasters. Lithium ions affect aquatic life, especially when present above trace levels. They disrupt the ion balances of sensitive organisms like fish and amphibians, which can lead to population changes down the food chain.
Isooctanoate, which is the tail end of the molecule, resists breaking down in the environment. It’s a hydrocarbon chain, much like what you’d find in some stubborn industrial pollutants. The real trouble here is persistence. Instead of breaking down quickly, these molecules can stick around in soil and water. Over time, that persistence sometimes turns small leaks into larger, system-wide problems. Think of classic pollutant cases like PCBs or PFAS—compounds we once called harmless or “inert” that we now regret releasing.
Making lithium isooctanoate isn’t a green process. Mining lithium takes freshwater resources and energy, and leaves behind salty waste. Add to that the solvents and byproducts from manufacturing the isooctanoate part, and you get a pretty messy signal. I’ve seen waste streams loaded with unusual organic acids and salts. When sites handle these without tight controls, the byproducts end up in rivers or landfills and keep seeping for years.
Waste greases and lubricants containing lithium isooctanoate add one last worry. People sometimes toss these down the drain or onto bare ground, not thinking that those small amounts slowly work their way into bigger environmental systems. Every summer, our local river sees tiny sheen patches during storms, likely caused by these “invisible” lubricant leaks. Local wildlife groups have tied these seasonal patterns to fish kills, especially in the hottest months.
So what turns risk into responsible practice? It really begins long before a product goes out the factory gate. Clean production technology can cut down on emissions and waste, turning the process from a dirty secret into something more sustainable. Manufacturers can invest in closed-loop water systems, better filtration, and safer solvents. On my end as someone who’s monitored compliance, strong government rules push lagging companies to do better, while industry leaders can work together to share safer alternatives and recycling schemes.
Handling and disposal also deserve real attention. Average folks and small businesses often miss the fine print on what to do with spent greases or old chemicals. The solution is straightforward: clear labeling, community take-back programs, and practical education go a long way. As with any emerging ingredient, transparency about the environmental fate pushes both industry and consumers toward smarter choices.
Lithium isooctanoate sits quietly on shelves in labs and factories, but ignoring its storage needs invites trouble. Few ever think about chemical safety until a problem arises. I’ve watched the damage that comes with overlooked basics, and even a detail like room temperature can make the difference between a regular day and a disaster report.
Lithium-based compounds often react to moisture, air, or accidental mixing with incompatible substances. A substance like lithium isooctanoate, with possible flammability and health risks, calls for more than generic advice—common sense and good habits keep everyone safe.
I’ve seen storage rooms in small labs and giant storage tanks at industrial plants. An overcrowded storage closet won’t cut it when dealing with lithium compounds. A dedicated, well-ventilated room, out of direct sunlight, gives a solid start. Clean air keeps vapors from building up. Closed containers stop contamination. Nobody wants to breathe in strange fumes at work.
Fire-proof cabinets become non-negotiable near anything with lithium in the name. You might shrug off the risk, but real-life chemical leaks and fire department calls say otherwise. Companies that store their chemicals away from heat sources, sparks, or even active electrical equipment sleep better at night. A fire marshal friend told me they spot more risks from poor storage than from actual mishandling.
Hot, stuffy rooms speed up chemical breakdown. Lithium isooctanoate fares best around regular indoor temperatures, kept out of humid zones or places with frequent drafts. Water and lithium compounds often spell bad news. I’ve seen cardboard storage warped by a leaky roof, with containers sitting in small puddles—no good can come from that. Sealed plastic or original packaging handles everyday bumps and splashes better than re-used jars or random plastic bottles.
Stickers fade and labels peel off, but guessing which bottle holds what doesn’t work for chemistry. Fresh labels—date received, hazard class, concentration—keep staff from mistakes. In one incident I remember, a missing label led to two nearly identical jars switched by a new hire. Mistakes often come from forgetfulness or improvisation during busy days.
Locked cabinets slow down accidents. Restricting access to employees with training shrinks the odds of someone dumping lithium isooctanoate into the general waste or rinsing it down a sink. Bodily harm from chemical burns or inhalation slips into news headlines all too often—not because the chemical changed, but because someone skipped steps.
Spill kits—absorbent pads, protective gloves, safety goggles—should sit right by the storage area. Signs with first-aid steps work better than thick manuals hidden in the office. I remember an emergency shower spending years clogged up by cardboard boxes, unopened. Walk through an emergency plan with real people. Make time for quick drills.
Disposal shouldn’t mean the trash or common sink. Partnering with hazardous waste services costs less than fines or cleanup bills. I urge anyone handling lithium compounds to check those details yearly. Regulations shift, and what flew last year might cause big problems now.
Safe storage takes more than checklists. Personal responsibility and real-world experience, backed by clear communication, keep both people and property unharmed. Paying attention to simple storage decisions shows more respect for staff health than any slogan ever could. At the end of the day, doing things right means everyone walks away in one piece, and that’s the best outcome any workplace can ask for.
| Names | |
| Preferred IUPAC name | lithium 4-methylpentanoate |
| Other names |
Lithium 6-methyloctanoate Lithium isooctanoic acid salt Lithium 3,5,5-trimethylhexanoate |
| Pronunciation | /ˈlɪθ.i.əm aɪ.səˈɒk.tə.noʊ.eɪt/ |
| Identifiers | |
| CAS Number | 68648-82-8 |
| Beilstein Reference | 1462465 |
| ChEBI | CHEBI:36897 |
| ChEMBL | CHEMBL4297372 |
| ChemSpider | 13498372 |
| DrugBank | DB14590 |
| ECHA InfoCard | 07dca085-7a8f-4f2e-961d-50513b90c03b |
| EC Number | 291-320-8 |
| Gmelin Reference | 113846 |
| KEGG | C14299 |
| MeSH | D02.241.081.150.500.600 |
| PubChem CID | 88644638 |
| RTECS number | OI9625000 |
| UNII | B7KS6N8K5D |
| UN number | UN3480 |
| Properties | |
| Chemical formula | C8H15LiO2 |
| Molar mass | 158.22 g/mol |
| Appearance | Colorless to light yellow liquid |
| Odor | Odorless |
| Density | 0.96 g/cm3 |
| Solubility in water | Soluble in water |
| log P | 2.8 |
| Vapor pressure | Negligible |
| Acidity (pKa) | pKa ≈ 4.85 |
| Basicity (pKb) | 8.88 |
| Refractive index (nD) | 1.423 |
| Viscosity | <500 cP at 25°C |
| Dipole moment | 2.6935 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 389.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -669.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -5697.7 kJ/mol |
| Pharmacology | |
| ATC code | N05AN01 |
| Hazards | |
| GHS labelling | GHS07, GHS08, Warning, H315, H319, H361 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H314: Causes severe skin burns and eye damage. |
| Precautionary statements | P260, P264, P271, P273, P280, P301+P312, P302+P352, P305+P351+P338, P310, P330, P337+P313, P362+P364, P405, P501 |
| NFPA 704 (fire diamond) | 1-3-0 |
| Flash point | 70 °C |
| Lethal dose or concentration | LD50 (Oral, Rat): > 2000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 >5000 mg/kg |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 100 mg/L |
| IDLH (Immediate danger) | N.D. |
| Related compounds | |
| Related compounds |
Sodium isooctanoate Potassium isooctanoate Calcium isooctanoate Magnesium isooctanoate Lithium octanoate |